Control Systems and Visualization
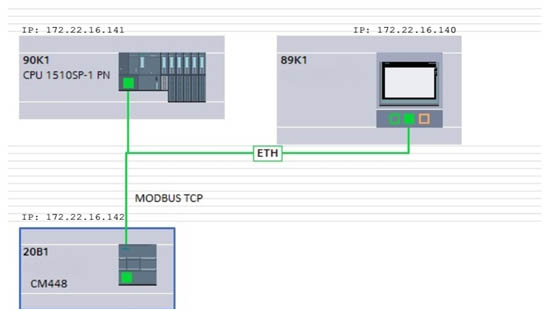
A properly designed and programmed control system is a prerequisite for the correct operation of any automated technological process.
The heart of the state-of-art control system is a programmable logic controller (PLC) along with digital and analog inputs and outputs cards. Digital and analog inputs are used to transmit information to the controller about the current state of the control devices of the process components, the measured values of the operating parameters and possible occurences of emergency conditions. Digital and analog outputs allow certain reactions to be triggered in process equipment connected to the control system, in accordance with a pre-programmed action algorithm or a command issued by the operator.
To ensure interactive communication between the system user and the PLC controller, a so-called Human Machine Interface is required. HMI which, depending on the complexity of the system, can be a set of buttons installed on the door of the control panel, a text display with function keys, a monochrome or color touch operator panel, or a panel or desktop industrial PC.
In case of pharmaceutical applications, HMI color touch panels in less advanced applications or industrial PCs with SCADA application installed (SCADA = Supervisory Control and Data Acquisition) in more complex applications are used as communication interfaces. Sometimes there happen combinations of both options when the operator panel is located directly on the devices and is remotely connected to the SCADA system installed on the computer located, for example, in the central server room. The SCADA system can be configures as a stand-alone or a client-server version.


Control systems used in the pharmaceutical industry should be designed, built, and tested in accordance with applicable GAMP guidelines, GMP Appendix 11 and CFR21PART11 requirements.
The GAMP Guidelines contain standards that define how to design, configure, and document computerized control systems used in the pharmaceutical industry. The main subject of CFR21PART11 requirements is to ensure the security, integrity and non-editability of data, including ensuring appropriate access levels for various user groups, as well as registering events in the form of “Audit Trail” records.