Pure Steam Generation And Distribution Systems
Pure Steam is used in the pharmaceutical industry is used to sterilize tanks and vessels and technological equipment, as well as to supply steam sterilizers (autoclaves).
Pure Steam is generated by evaporation of Purified Water or Water for Injections in pressurized conditions. The quality of Pure Steam used in the pharmaceutical industry is determined by examining its condensate parameters. The quality requirements for Pure Steam condensate are similar to those for Water For Injections.
In addition, if Pure Steam is used in steam sterilizers, then its physical parameters must comply with the requirements specified in EN 285 parameters. The most important are as follows:
|
Parameter |
Quality Requirement |
|
Non-condensing gases content |
≤ 3.5% |
|
Dryness value |
≤ 0.95 |
|
Degrees of superheat |
≤ 25 K |
To achieve the quality parameters of Pure Steam that are compatible with the requirements of EN 285, it is very important to properly prepare the feed water for the pure steam generator, including its degassing. There are two methods to carry out degassing: thermal method or a membrane contactors.
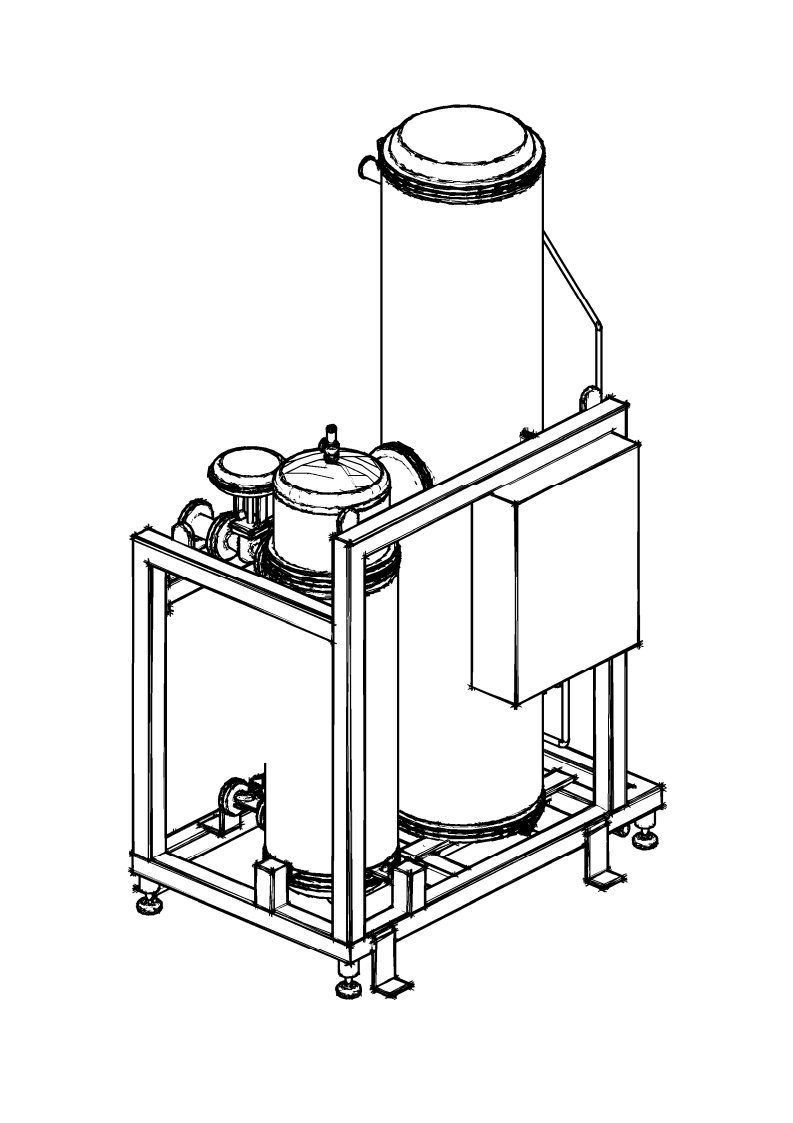
The properly designed Pure Steam distribution system is not less important than the generation system. The task of the distribution system is to deliver Pure Steam to all user points under the appropriate pressure and at the required temperature, without compromising the quality attributes. The Pure Steam distributing system should be designed in a way to minimize energy losses and guarantee effective condensate drainage, eliminating the risk of air and gas accumulation.
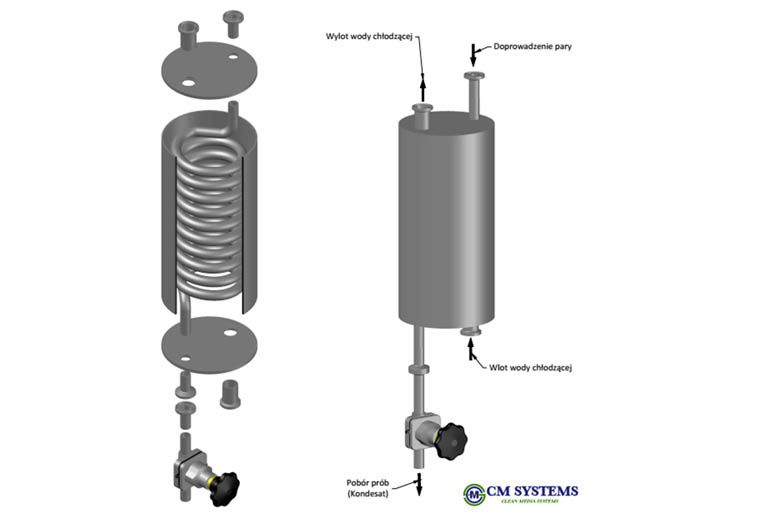
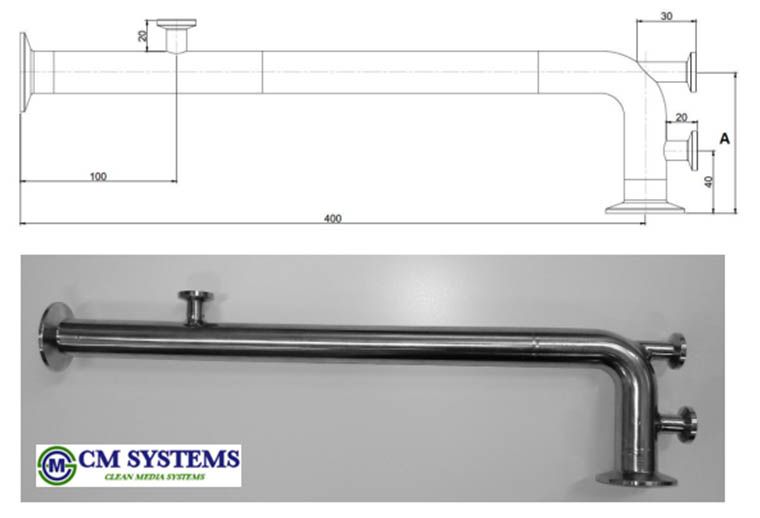
In the most distant and neuralgic branches of Pure Steam distribution systems, usually there are sampling coolers and EN285 test elbows installed in order to sample Pure Steam condensate and to control its physical parameters for the compliance to EN 285.